New Year’s Eve is approaching and with it the uncontrolled detonation of dangerous explosives. But they light up colourfully and bang loudly, so we don’t care for this one night of the year. But how do the colours get into the fireworks? There is actually quantum physics hidden in something as mundane and huge as a firework display.
19 shapes of ice
Let’s start at a very basic level: with fire. I am actually not surprised that people in Ancient Greece and even beyond the Middle Ages believed in the doctrine of the four elements. Everything is made up of the four basic elements: Earth, water, air and fire.
They stand for different phases of matter: the solid, the liquid, the gaseous and – obviously – the glowing. In principle, this is not so stupid, because we know something very similar from many (chemical) elements, such as water. It can be liquid, solid and gaseous. However, water rarely burns.

We now know that matter is made up of molecules, which in turn are made up of atoms. Depending on how the atoms are arranged, the substance is, put simply, solid, liquid or gaseous. This is simplified because many substances can assume more than these three states of matter. In fact, ice can assume 19 known phases. Depending on the pressure and temperature, the molecules arrange themselves in different geometries.
The stuff that burns
But what actually is fire? Is it also made up of atoms? In the 17th century, people believed that a mystical substance, a volatile substance that burns in the form of a flame, escaped from all burning materials. They called it phlogiston, from the Greek word for “burnt”.
This was mainly based on the observation that many substances become lighter when they burn, for example, wood or paper. Other substances, however, became heavier, which didn’t quite fit the picture. But that didn’t bother anyone too much, chemistry was little more than advanced alchemy back then.

The German-Swedish pharmacist and chemist Carl Wilhelm Scheele wanted to find out more. In 1772, he set fire to various substances and tried to produce phlogiston. He heated various substances in sulphuric acid and discovered a colorless and odorless gas that he could ignite. He called it fire air.
The English chemist and theologian Joseph Priestley carried out similar experiments somewhat later and called Scheele’s “fire air” “phlogiston-free air” himself. Because he published his results three years before Scheele, Priestley took credit for the discovery – which was a shame for Scheele.
However, neither of them knew that they had discovered a new element: Today we know fire air as oxygen. This was first discovered by the French chemist Antoine de Lavoisier. He assumed that the element was the basic component for the formation of acids (but it is not, it is hydrogen), so in 1779 the element was given the name “oxygenium”, which means acid former – oxygen.
What is fire?
So there is no fire air, but what exactly is fire? We can see it, but we can’t touch it, and it’s very hot. What is needed for a fire is a combustible material, for example wood, oxygen, and a sufficiently high temperature.
Fire is the visible result of combustion. This in turn is a chemical reaction in which a combustible substance oxidizes with the help of oxygen and energy is released. In short, fire is the energy that is released during a certain form of chemical reaction. This is much more abstract than “matter is made up of little balls” – I’m not surprised that fire has fascinated people in the past as much as it does today.

Fire is not only hot, but we can also see it. A flame forms because gases are released during combustion, these rise upwards – because they have a lower density than air – and the typical flame shape is formed. Because the difference in density does not play a role in weightlessness and the gases do not rise upwards, flames in space do not have a droplet shape but are spherical.
Why can we see fire?
However, something almost always remains after combustion and in a campfire or candle this is soot. This consists of carbon. These small carbon particles float to the top and start to glow in the heat – and that is what we can see. The reason why they glow orange-reddish is blackbody radiation (more on this here). At a flame temperature of just under 1200 to 1400 degrees, this has an orange colour.
However, this is not the only reason why we can see fire. In a Bunsen burner, for example, it is not carbon that burns, but propane or butane – gases that consist of hydrocarbon chains, i.e. carbon (C) and hydrogen (H) atoms strung together. At “low” heat of around 900 degrees, combustion is incomplete and the resulting soot particles glow.
At full heat of approx. 1500° degrees, however, the flame is roaring and blue. This flame is called non-luminous because the gas is completely burnt and soot particles do not glow. The carbon-hydrogen chains break up in the heat. The reason why these CH compounds glow blue is finally hidden in quantum physics!
A colourful quantum leap
Atoms are made up of protons, neutrons, and electrons, and – as we already know – electrons can only orbit around the nucleus of an atom in very specific orbits. They cannot fly between two orbits, but can only hop from one orbit to the next. To hop to a higher orbit, they need energy – for example, the energy released during combustion.
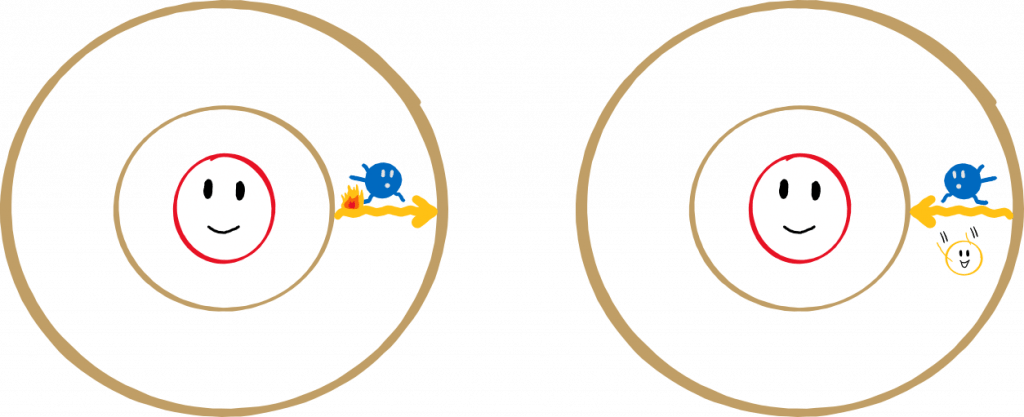
However, the electrons don’t like it very much on the higher orbits, and at some point they hop back onto a lower orbit. This releases a certain amount of energy, which is characteristic of the atom. The energy is released in the form of packets – photons – and depending on the amount of energy, they glow in different colours. Large energy packets are “bluer” while smaller ones are “redder”.
Why are fireworks colourful?
The CH molecule emits light with a wavelength of 432 nanometers, i.e. blue light, as we can see in the Bunsen burner. In other elements, the distances between the lanes are different and the flame glows in a different colour.
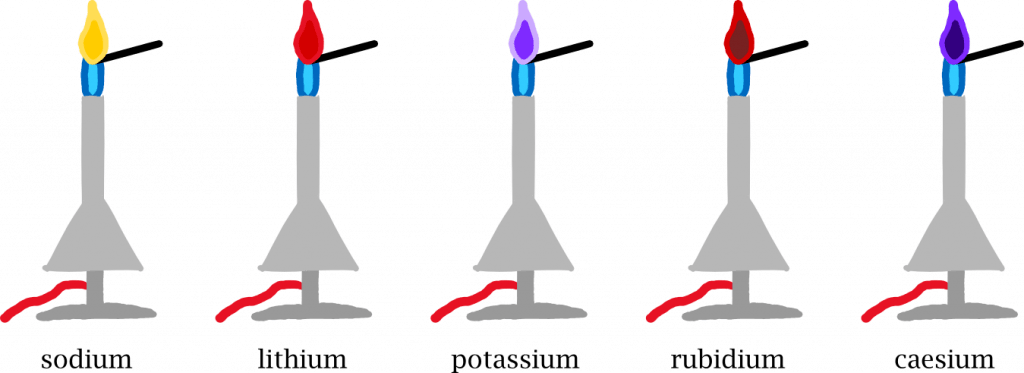
The element rubidium burns red, caesium blue-violet. Chemists use this to identify elements. Pyrotechnicians use it to create colourful fireworks. If they mix copper into the explosive device, they get emerald green explosions, common salt colours them yellow, potassium purple and strontium red.
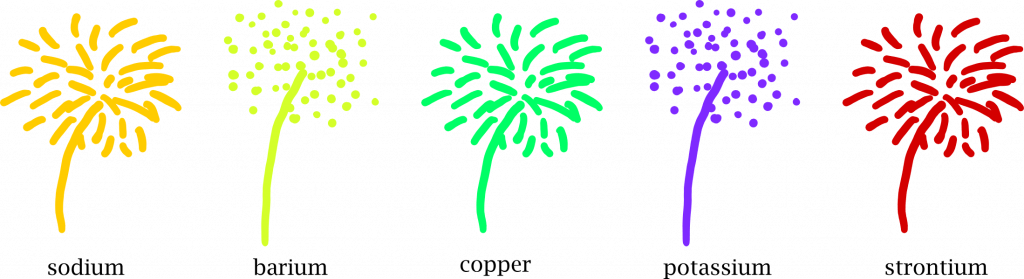
So the next time you see fireworks, you can try to guess the elements and think of the bouncing electrons that are set on fire at every firework display.
Do you like what you read? Then you can buy me a coffee here! And if you don’t want to miss any new posts, don’t forget to subscribe to my blog.
