Quantum technology is on everyone’s lips. Boulevard newspapers report on quantum computers under the factual title “Computers will dominate mankind!” In most cases, quantum technology is presented mysteriously, as a product of the future: science fiction. But one point most articles keep quiet about: Quantum technology already exists and we all have it at home.
Quantum theory was born at the beginning of the 20th century, when Max Planck dealt with blackbody radiation and Albert Einstein with the quantisation of light. They discovered – in a long process and together with many other physicists – one of the most fundamental concepts of quantum physics: the wave-particle dualism.
The wave-particle dualism states that particles sometimes behave like waves, and that waves sometimes behave like particles. Light, for example – long believed to be a wave – sometimes behaves like a stream of solid balls. Electrons – which we learned in school were particles – behave like waves in some experiments. But wave-particle duality is bigger than just saying “it’s sometimes like this, and sometimes it is different”. It means that objects in quantum physics can no longer be categorised into “waves” or “particles”. This has far-reaching consequences: for light, electrons, atoms, and also for our everyday lives.
Sunny electricity and glowing barriers
Einstein discovered that light consists of particles – photons – and was thus able to explain the photoelectric effect. It says that you can release electrons from a metal if you bombard it with light of the right colour. And electrons in motion are nothing other than electricity! So from here, only a few steps were missing to the development of the solar cell. In the 1940s and 1950s, employees at Bell Labs succeeded in turning sunlight into electricity. Over the decades, this developed into one of the most important renewable energy sources.
Alongside this milestone are small technologies that we know from everyday life. Light barriers in supermarkets, photocopiers, CCD sensors in webcams, and night vision devices. They are all based on the principle that light can trigger a flow of electricity. Praise be to photons, because who would want to open the supermarket door themselves?
Electron clouds and silicon valleys
The realisation that electrons behave like waves leads to a completely new view of matter. Atoms – the building blocks of matter – consist of a tiny atomic nucleus and electrons flying around it. Until the 20th century, people thought of an atom as a small solar system: Electrons orbiting around the atomic nucleus, like planets around a sun. But since electrons are not actually particles, this image had to be abandoned. It was discovered that electrons are rather smeared around the atomic nucleus like a cloud. Admittedly, very strangely shaped clouds. We can no longer say where exactly the electron is, only that it is somewhere in this cloud. We call these electron clouds orbital, in reference to the orbit of a planet. This new atomic picture has been an eye-opener for scientists in many ways. They suddenly understood the periodic table, chemical bonds and the discrete energy jumps in atoms.

If you put many atoms together in an orderly way, you get a solid; i.e. crystals, metals, salts, shopping centres, all such things. Densely packed atoms, however, behave differently than lonely ones. Their orbitals overlap, which leads to fascinating effects. For quantum technology, the emergence of semiconductors has a special significance. Semiconductors are temperamental hybrids – half conductor, half non-conductor. If they are too cold, they do not conduct electricity. But if you heat them up properly, their conductivity increases. The most famous of the semiconductors is silicon. This should ring a bell because Silicon Valley was named after it. The home of giants like Apple, Intel, Google, Facebook and Tesla. Silicon is the main ingredient of computer chips and transistors, and thus the basis for microelectronics, computers and smartphones. Silicon has probably influenced our way of life as much as stones influenced the Neanderthals. So much that our time is also called the silicon age.
Sociable photons and climbing goats
It had already been established before the development of quantum theory that an atom can only absorb or emit very specific amounts of energy. This means: the energy levels of the atom are discrete. But it was only with quantum theory and the orbital picture that this observation could be explained properly. The energy of the light you shine on the atom must correspond exactly to the energy gap in the atom, otherwise you can’t excite it. And if you get excited, you have to calm down at some point; this also applies to atoms. At some point, the atom will release the excess energy in the form of a photon with a very specific energy to return to a more relaxed state.
If you bring a lot of atoms into an excited state at once, something exciting happens. Photons are social animals, and when one atom releases a photon, all the others join in. All these atoms release identical photons that march in step in the same direction. And that is nothing other than a laser! Incidentally, this is an acronym that signifies ‘light amplification by stimulated emission of radiation’.

By the way, such acronyms don’t come out of the blue but are hard work. At some point, researchers have to sit down and create a cool acronym for their research. In my research, I work with optimisation algorithms that have magnificent names like GRAPE, GOAT, and CRAB. GOAT, for example, stands for Gradient Optimisation for Analytic conTrols. Someone tried really hard to come up with a great acronym there. By the way, the GOAT algorithm is not to be confused with the Wild Goats algorithm, which deals with the climbing behaviour of goats.
Let’s get back to lasers. They are the celebrities of physics and they are everywhere. In obvious places like laser pointers, laser printers and in laser shows, for one. But they are also hidden in many important technologies in everyday life and medicine. CD, DVD and Bluray drives, material processing, measurement technology, cancer therapy and laser surgery would not work without lasers.
Accurate clocks and many satellites
The discrete energy levels in the atom are not only important for lasers but also for the atomic clock. The exact spacing of atomic energy levels corresponds to precise frequencies of light and thus precise units of time. You often hear impressive comparisons, for example that an atomic clock would only be off by one second since the Big Bang. But what do you actually need such an accurate clock for? Probably not for cooking eggs.
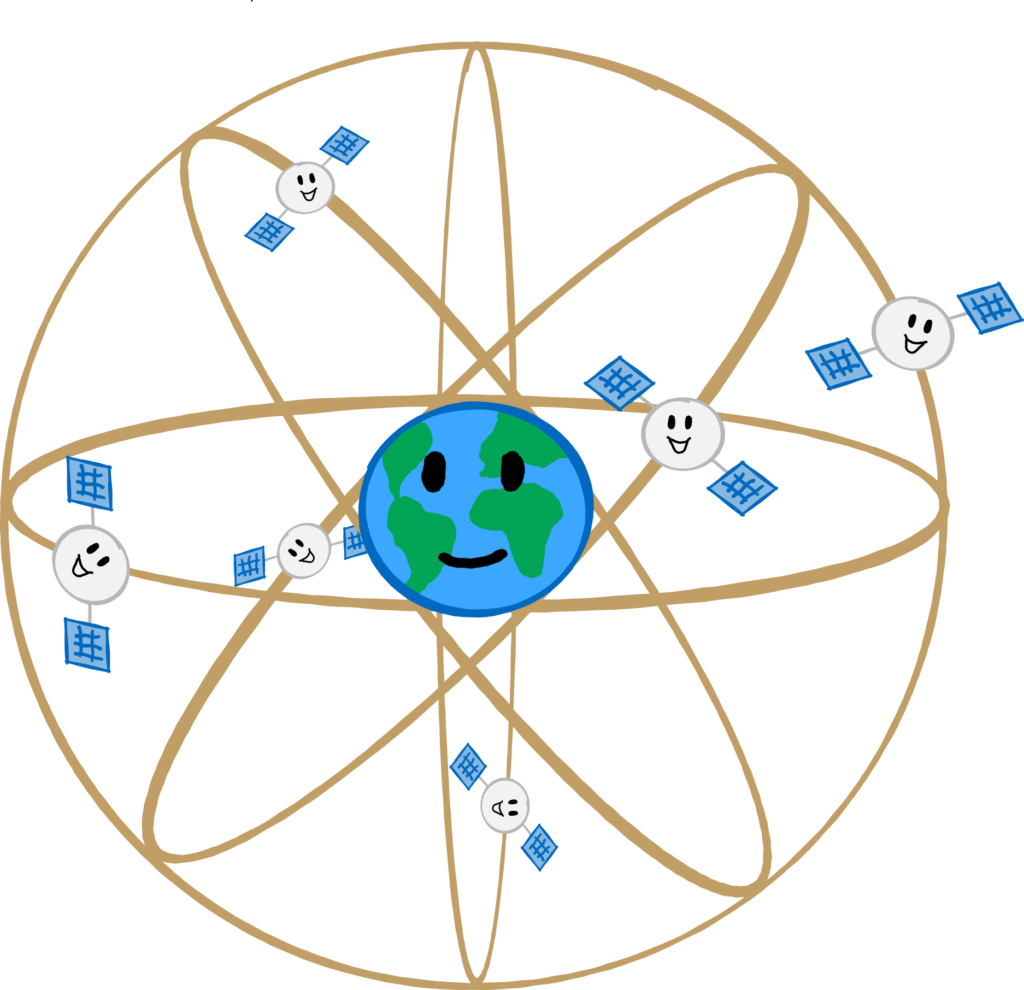
We all have the answer in our pockets: for the smartphone, or more precisely, the GPS. Again, an abbreviation; it stands for global positioning system. It was originally set up by the US Department of Defence. (What more can I say?)
For GPS, 24 satellites orbit the earth in six different orbits. If I want to determine my location, my phone sends a signal to the GPS satellites. From the distance between me and three or more of these satellites, it is possible to calculate exactly where I am on Earth. For greater accuracy, the time it takes for the signal to travel from me to the satellite and back is also calculated. Since light is very fast, this requires very accurate clocks, which is why each of these satellites is equipped with an atomic clock.
As a normal human being, GPS tells you your location with an accuracy of about 8m. However, there is something like GPS+, which is reserved for the military alone (PPS, Precise Positioning Service). With this you can already achieve an accuracy of 6m. Using various correction methods, this value can be improved to an impressive 10cm. Useful if someone in the military has lost their mobile phone in the forest.
The First Quantum Revolution
All these technologies can only be fully explained with the help of quantum physics. This distinguishes them from “conventional technology”, which we can understand with the help of classical physics. A car, for example. They have led to the so-called first quantum revolution. From the 1940s to the 1960s, a staggering amount of new technology emerged that changed our lives.
As the name “first quantum revolution” suggests, we have not yet reached the end of the journey. Today, less than a hundred years later, we are in the midst of the second quantum revolution – a revolution of superlatives: smaller, faster, safer, precise..er. A revolution in which the big players are involved: Google, IBM, Microsoft, the German railway system. And this is the technology that is meant when newspapers report about “quantum technology” these days.
However, two revolutions in one day is too much, and so I will devote myself to the second quantum revolution in my next post. Until then, you can pass the time with your smartphone, a laser show, or a good old DVD, and remember the quanta to which we owe all this.
Do you like what you read? If you don’t want to miss any new posts, don’t forget to subscribe to my blog.
Sources:
Jonathan P. Dowling, Gerard J. Milburn – Quantum technology: the second quantum revolution.
Wild Goats Algorithm: An Evolutionary Algorithm to Solve the Real-World Optimization Problems
GOAT algorithm: Tunable, Flexible, and Efficient Optimization of Control Pulses for Practical Qubits