Would you say you would have behaved differently if you knew you were being watched?
Eamon Bailey in The Circle
I recently read the book “The Circle” by Dave Eggers. It’s about a Facebook-Twitter-like Internet company called “The Circle” that wants to create complete transparency and networking – privacy, unfortunately, falls by the wayside. The quote above is by one of the Circle bosses and he uses it to justify his ubiquitous surveillance.
I am sure that we would all answer this question affirmatively. We behave differently under surveillance than when we are alone. So does that mean that pure observation influences our behaviour?
The Observer Effect in Psychology
Psychologists have long known about and researched the effect that people behave differently when they know they are being observed: It is called the Hawthorne effect. It was first studied in the 1920s and 30s by U.S. scientists at the Hawthorne factory.
Put simply, we want to evoke a certain reaction in others when being watched: We usually behave better than when we are unobserved. But “unfortunately” this is not an absolute causal relationship: you don’t become 10% more productive or 20% cleaner by being observed. This makes it insanely difficult to study human behaviour.
The Observer Effect in Quantum Physics
In quantum physics, the answer is simple. The pure act of observation – or more precisely “measurement”, because “looking” is merely a way to measure something – generally changes a quantum system. There are various examples of this.
The Moon
Well, not the moon itself, but a very famous quote:
Does the moon also exist when no one is looking?
Bohreinsteisenberg
No one knows who and if anyone ever said it. Albert Einstein? Erwin Schrödinger? Niels Bohr? The background was probably a thought experiment – typical for all three – and the question: Does an object possess a certain property, independent of whether it is observed or not? That is, regardless of whether someone measures the property?
In our everyday world, this question seems absurd: a bar of chocolate always weighs 100 g, whether I put it on the scale or not. In the quantum world, however, we (here on this blog) have often seen that quantum properties are not set in stone to the same degree.
The best example is superposition: a quantum object can be in a superposition of two states, for example, “up” and “down”. In this case, the pointer position “up or down” is not defined. Only when we measure (observe!) the state of the qubit, it decides for one of the two options and only then the value is defined. Which value is measured is given by a probability distribution.
The way to the uncertainty relation
What has the moon to do with it? Well, qubits were not yet known to Bohreinsteisenberg. Instead, they looked at particles in cloud chambers: Boxes of supersaturated water vapour in which radiation becomes visible. When a particle whizzes through the cloud chamber, a clearly visible contrail forms – much like a subatomic aircraft.
The trails are clear stripes and visualise the path of a particle, similar to the orbit of the moon. However, physicists in the 1920s understood that electron orbits could not be determined at all, but only their approximate location in an orbital – an electronic cloud – around the atomic nucleus. Only when the electron is observed does it settle on a location. How do both fit together?
Some sources claim that Einstein – the eternal critic of quantum mechanics – thought: Not at all. He sneered, whether the moon is also not there like an electron if nobody looks at it. This is said to have moved Heisenberg to establish his famous uncertainty principle, which solved the problem: the location and velocity (or, to be precise, the momentum) of a particle can only be determined with a certain accuracy. The more precisely one is known, the more inaccurate the other becomes.
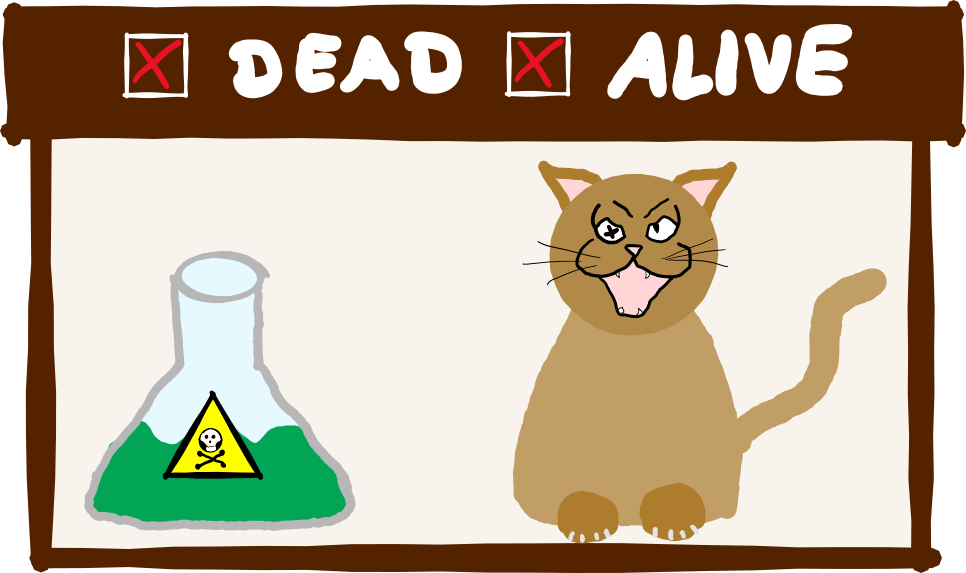
Schrödinger’s cat
Schrödinger’s cat probably needs no introduction. Erwin Schrödinger formulated this thought experiment in 1935 and put his cat in a box with a poison bottle and a radioactive compound. If the atom decays, the bottle breaks, and the cat dies; if not, it doesn’t – you know the story (if not, you can read it again in detail here).
The trick is: Whether the radioactive atom has decayed or not – and whether Schrödinger’s quantum cat lives or dies – is only decided when we open the box. In other words: Only as soon as someone looks at it. That sounds absurd, and it should. Because actually, Schrödinger wanted to take the observer-dependent measuring process ad absurdum with his thought experiment.
Schrödinger’s cat has another layer of complexity: Namely, there are no superposition zombie cats. This can also be resolved with the observer effect, but in a somewhat more complex form: decoherence. Not only do we humans observe the cat. Also, the air, the light, and the dust “observe” it. The superposition is destroyed faster than it can be formed, and Schrödinger’s zombie cat becomes the boring (but famous) house cat.
Double-slit experiment
The double-slit experiment was the first experiment I learned about at school in the introduction to quantum mechanics. At first, I thought: Yes, of course, it’s perfectly logical. Later I realised that I didn’t get it. Only then did I understand how bizarre light really is.
Step 1: Light waves
Let’s start with the simplest, non-quantum mechanical case: Let’s think of light as a wave, as scientists have long done. When we shine light on an aperture with two slits, a pattern of stripes appears on the screen on the opposite side because the crests and troughs of the waves alternately amplify and cancel each other (more on this here).
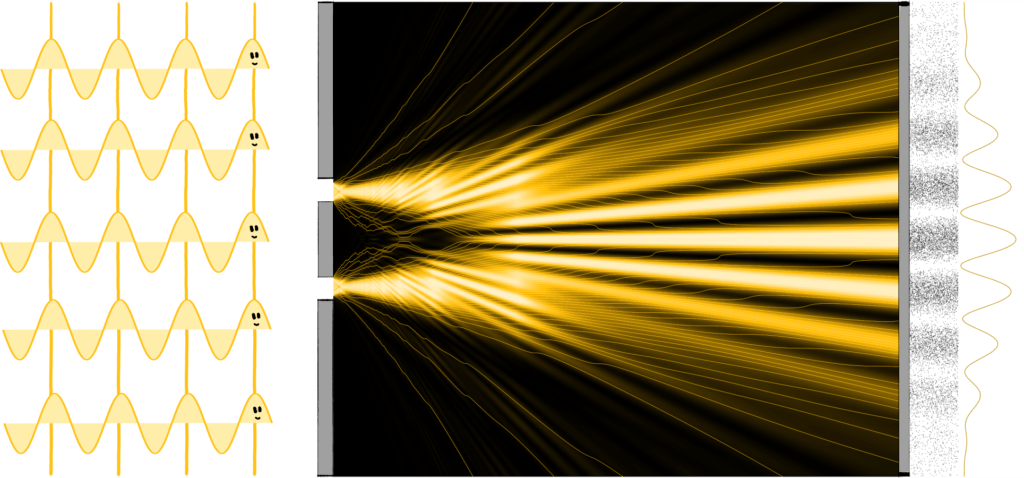
Step 2: Classical particles
Let us now imagine that we would do the same experiment not with light waves, but with particles. Think, for example, of a paint spray can, of a stencil of a graffiti sprayer. In that case, we don’t see a stripe pattern on the right, but two thick splotches directly behind the slits.
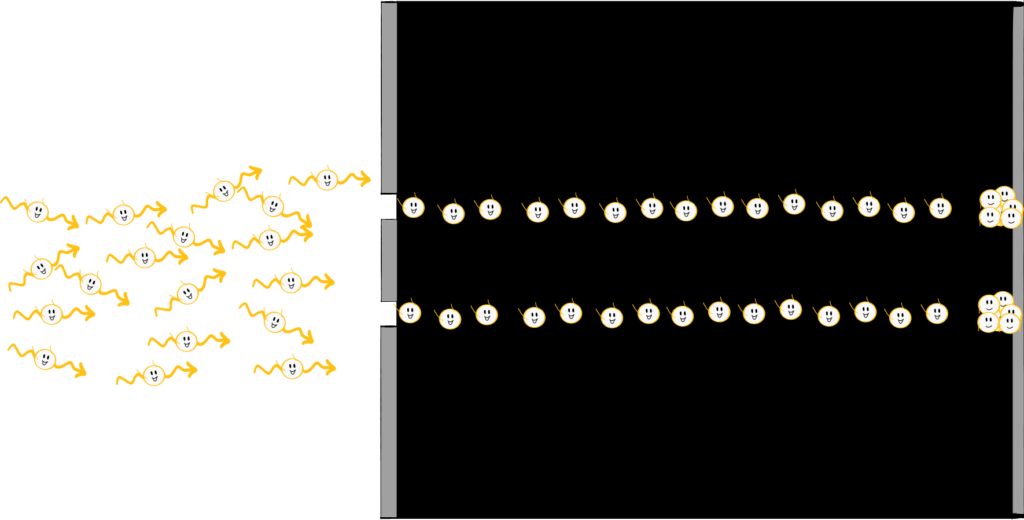
Step 3: Photons
Now we turn quantum mechanical. We know by now (already since about 1900) that light is built up of small energy packets, the photons. What happens when we shoot single photons at the double slit? Do they behave like waves? Or like classical particles?
The groundbreaking discovery was that when light is shot one photon at a time at the double slit and the points are recorded, a fringe pattern gradually appears on the right side of the screen – exactly the same as if a beam of light had been emitted directly. Every single photon has a wave character and interacts with itself – this is what we call wave-particle duality.
Step 4: Which path does the photon take?
So that the stripe pattern can develop, the photon must go through both slits at the same time and interact with itself. But this is absurd – it must fly through either the top or bottom slit!
Clever as the physicists of that time were, they thought: Let’s build a detector into the aperture and see through which slit each photon passes. The spooky thing is: As soon as we register which path the photons take, the stripe pattern disappears. All that remains are two splotches directly behind the slits, just as with classical particles. The measurement breaks the quantum property of the light.
These are just three examples of how observation – or measurement – affects an object in quantum physics. In short: enormous! Why did I initially stretch so far and venture into psychology? I was speaking about this phenomenon in a popular science lecture and the question that came from the audience was: “Psychology also came up with the realization that observations affect human behaviour. Was that a trend then?”
Schrödinger’s cat and similar considerations of the observer effect in quantum physics emerged in the 1930s. The experiments on the Hawthorne effect were done from 1927 to 33. That is actually very close. I personally don’t think that one was inspired by the other there. Nevertheless, it’s an interesting trend.
Looking can make a big difference – I think that’s insane. Something as banal as measurement can shake up our view of the world. That’s why I’ve been looking at it so closely in my research. And that’s why I want to dig deeper into it in upcoming posts, showing you how measurements can affect everything, enable quantum technology, and even freeze motion. Stay tuned: Quanta are watching you!
This is the first part in the series “Quanta are watching you”. To make sure you don’t miss a post, don’t forget to subscribe to my blog. If you like what you read, you can buy me a coffee here!