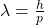Sometimes you have to make a choice. Some things you hate, for example, or you love them. Like Brussels sprouts, marzipan or Big Brother. In other cases, you have to take sides: Cats or dogs, Edward or Jacob, wave or particle.
But what if you just can’t decide? I have a friend who has two cats and a dog. Most people throw up their hands in horror here: That’s not possible! You can’t be a dog and a cat person!
Apparently, it is possible. Sometimes you can be two things at once. But when this happens in the very foundations of physics, it can start heated discussions. Like at the beginning of the 20th century when Albert Einstein threw light into a deep identity crisis: wave or particle, which is it?
From visual rays to light rays
Light is everywhere. Even in the middle of the night, the sky is still illuminated by stars and the moon. But what actually is light? You can’t touch it, you can’t smell it, you can’t hold on to it (or at least only with great difficulty) and it is almost as difficult to lock it out. As early as antiquity, Pythagoras asked himself the question of how we see. He believed that “visual rays” emanate from our eyes which objects reflect back. Later, however, it was discovered that light does not emanate from the eyes but from the objects themselves, and that light rays strike our eyes. In the 17th century, things finally became more concrete, and two fundamentally different approaches emerged.
Isaac Newton held the view that light consisted of particles, which he called corpuscles. This theory can be used to explain the reflection of light, for example. Reflection is what you can observe in a mirror or on a water surface: The light of the sun is reflected by the surface of a lake, so that you can see the sun in the lake without having to look at the sky. If light consists of particles, this can be explained quite simply by particles bouncing off the surface like bouncy balls.
However, light is not always completely reflected off a surface. For example, when a beam of light enters water, the angle it makes with the surface changes on the other side. This effect is called refraction and can be observed on a straw in a glass of water: viewed from the side, it looks as if the straw bends exactly where it passes through the water surface. This is difficult to explain in the particle picture of light but Newton went to great lengths to defend his theory.
Reflection and refraction, however, can be explained without much contortion by the assumption that light is a wave. The wave theory of light was coined in particular by the Dutchman Christiaan Huygens (whose surname and pronunciation are among the great unsolved problems of physics). But even physics is not safe from popularity contests, for despite the elegance of the wave theory, Newton’s corpuscle theory had prevailed at first.
The double-slit experiment
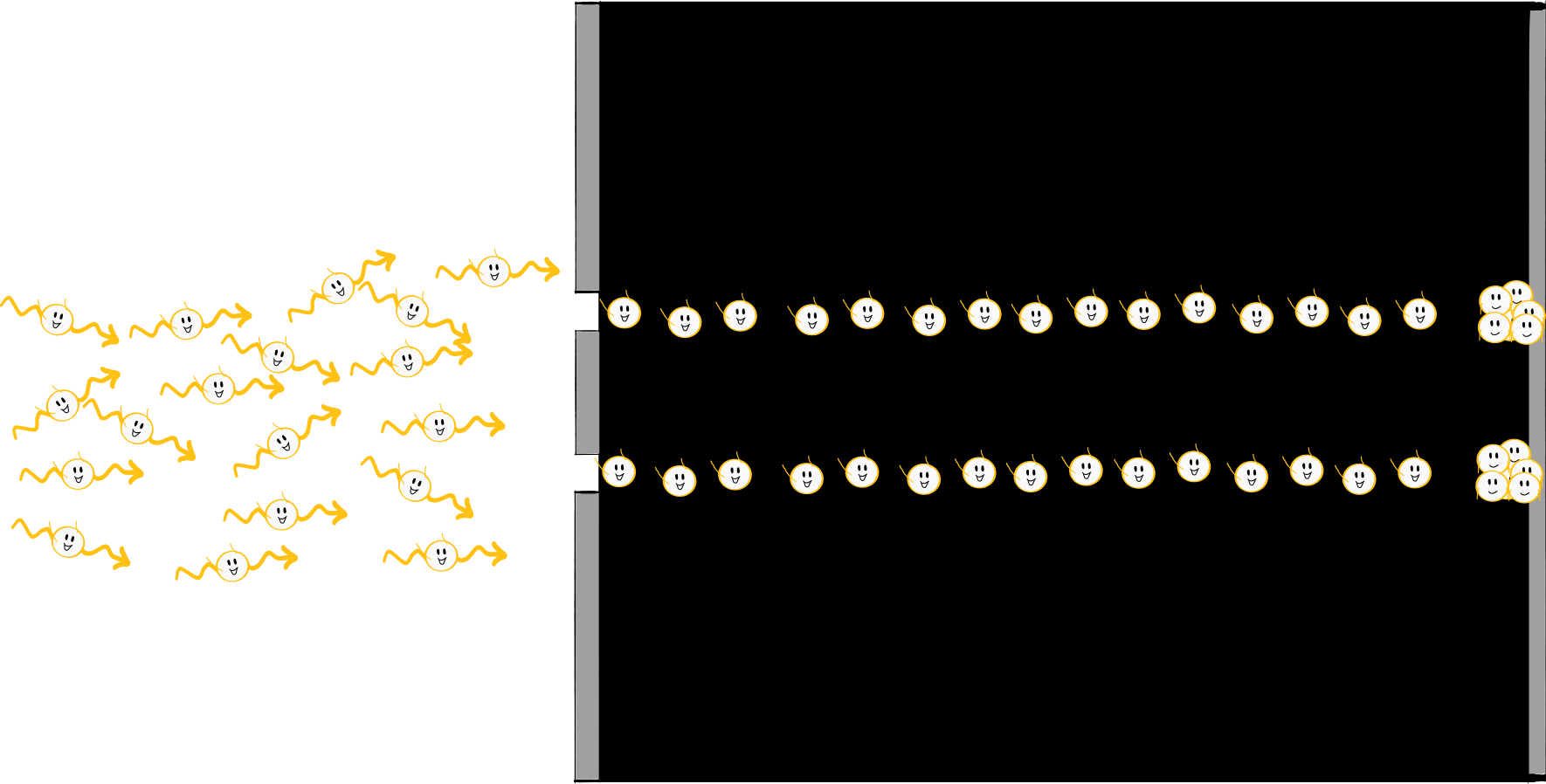
Newton’s corpuscle theory had its weaknesses and the experiments it could not explain became more frequent. The decisive factor in its downfall was the double-slit experiment carried out by Thomas Young in 1802. He shone light on an aperture with two slits (the double slit) and voilà, a fascinating pattern of light and dark stripes appeared on the opposite wall. A simple, pretty, and at the same time groundbreaking experiment because it can only be explained by the wave theory of light.
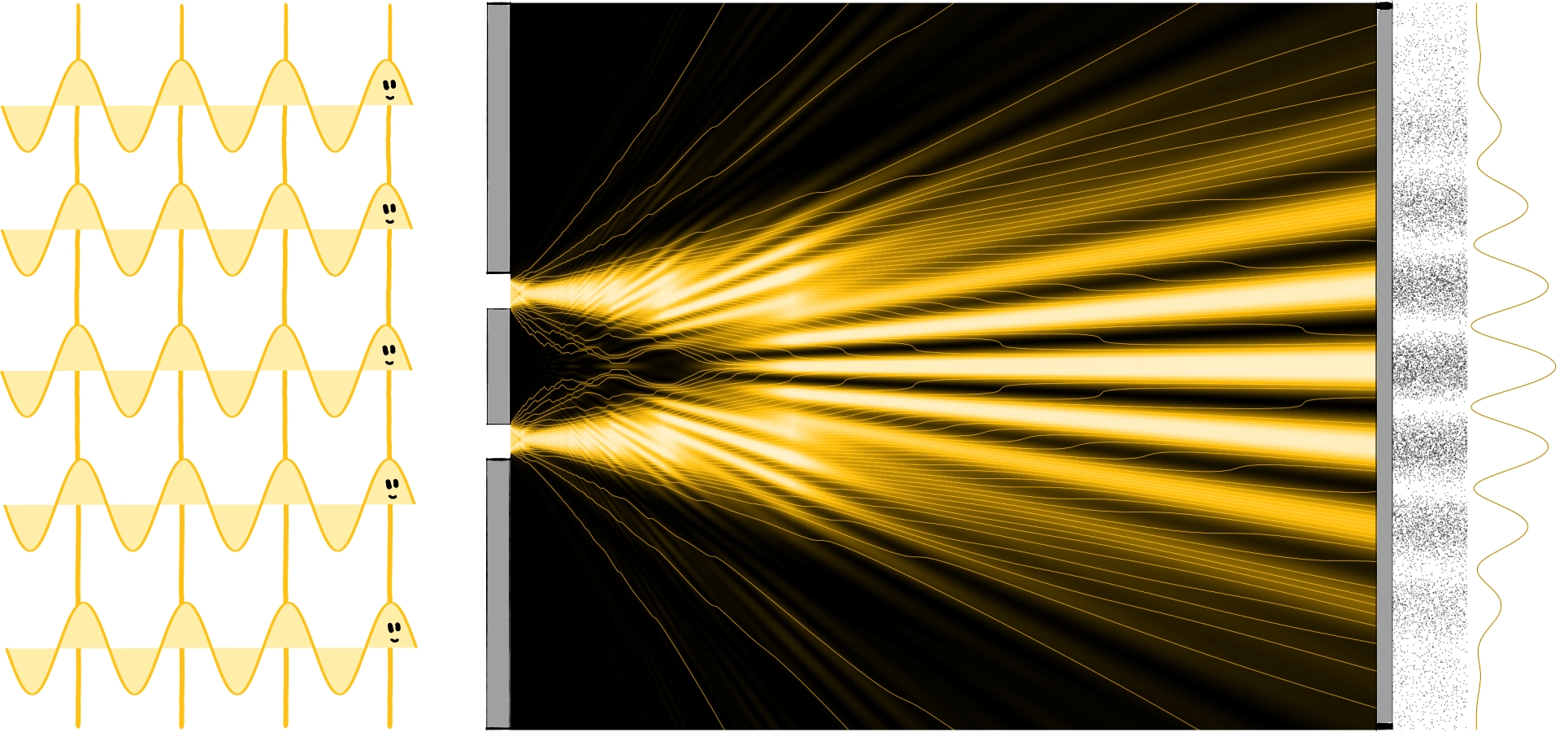
An important difference between waves and particles is the way they interact with their fellows. Particles are generally not very sociable: if several particles meet, they bounce off each other like billiard balls and go their ways. Waves, however, influence each other and can overlap – we call this interference. There are two extreme cases: if two wave trains harmonise and oscillate in the same way, they amplify and support each other. The two waves merge into one wave with twice the amplitude. The technical term for this is constructive interference. We know this term mainly from constructive criticism, which is also supportive and beneficial. The other extreme is less friendly. If the two wave trains are opposed to each other, they cancel each other out. The result of destructive interference is the destruction of the waves. So, much like destructive criticism, the result is the complete annihilation of the subject.
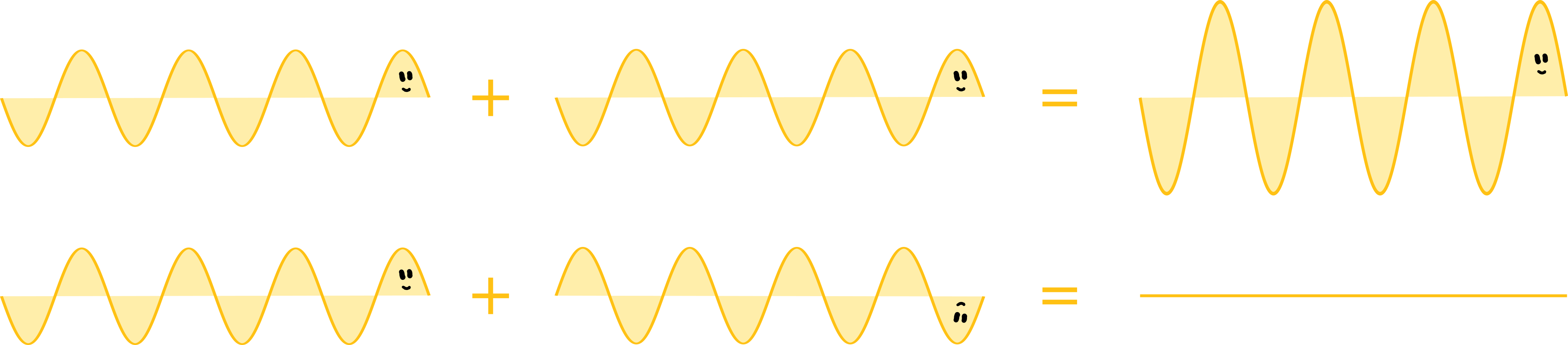
The two slits of the aperture of the double-slit experiment separate the laser light into two identical waves that overlap between the aperture and the wall. The fringes on the wall are created by alternating constructive and destructive interference. But this only happens if light is a wave because particles would behave significantly differently. For example, if you shoot balls at a (very large) double slit, you will find a bunch of balls behind each of the two slits. Assuming you shoot without aiming very hard (be it due to lack of intention or lack of talent), you happen to hit sometimes one slit, sometimes the other, and you have two roughly equal-sized piles behind the screen. But definitely no striped pattern.
The final crisis of light
Young’s experiment seemed to settle the matter once and for all: Light is a wave. Young spent his final days with the certainty that light waves were coming out of his candle. But the peace did not last long because just a hundred years later the wave picture was once again challenged. The triggers were Max Planck and Albert Einstein, who discussed two experiments that were incompatible with the wave picture of light – they needed the particle model. So back to square one? It’s not that simple because, of course, you couldn’t just ignore the other experiments that could only be explained by light waves. Maxwell, for example, had just shown that light is an electromagnetic wave. Was that simply wrong after all? Or did Planck and Einstein get it wrong?
Einstein proposed a theory that had never existed before. Perhaps he was a follower of Sherlock Holmes and followed his maxim:
When you have eliminated the impossible, whatever remains, however improbable, must be the truth.
Sir Arthur Conan Doyle, stated by Sherlock Holmes
Light must be a particle. But light must also be a wave. What do we conclude from this, Watson? Light must obviously be both.
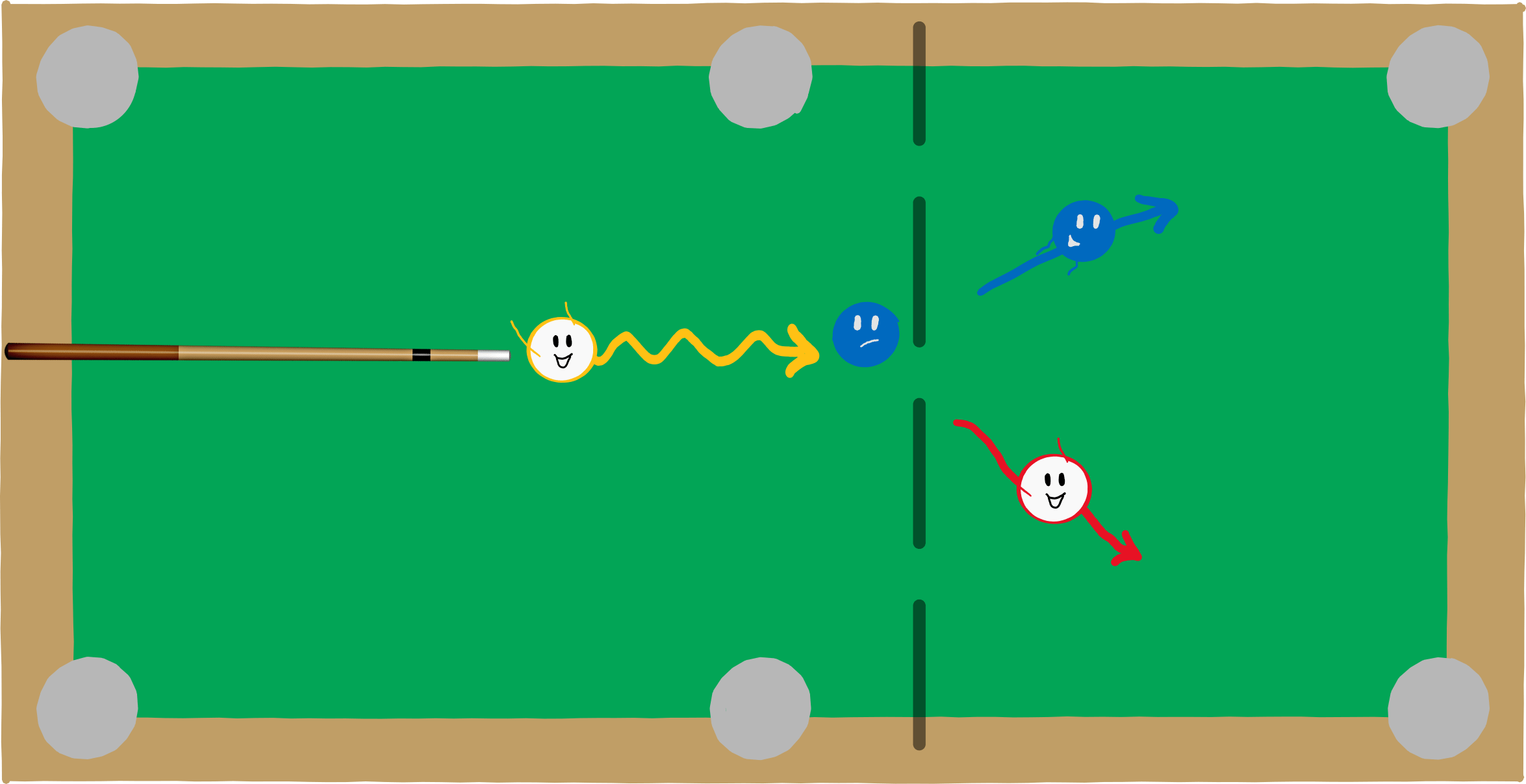
This assertion went (and still goes) against all intuition. But the evidence piled up. In 1922, Arthur Compton conducted another experiment that indisputably showed the particle character of light. In his experiment, he shot photons, i.e. particles of light, at an electron at rest. He found that the two particles behave in principle like billiard balls: If you shoot a photon at an electron, the photon gives some of its energy to the electron and both particles move away in different directions. This billiard ball behaviour is typical for particles – waves would behave quite differently when they collide. The particle character of light was then considered indisputable and Compton was awarded the Nobel Prize in 1927 for the discovery of the Compton effect.
Wave-particle dualism
They tasted blood. Everything seemed possible! If light, which we thought was a wave, is now suddenly also a particle – are particles then possibly also waves? The answer is as astonishing as it is simple: yes.
The Frenchman Louis-Victor de Broglie was the first to translate this assertion into a mathematical formula:
De Broglie already formulated this formula as a PhD student. It is the subject of his doctoral thesis with which he won a Nobel Prize. Just like that. This dissertation has become a small myth in physics: Our professor told us at some point during a lecture that de Broglie’s doctoral thesis was only three pages long because the simple elegance of his formula did not require any further elaboration. That sounds nice, but unfortunately, it is not true. All in all, his thesis is a good hundred pages long and contains more than just this one formula.
But de Broglie’s equation was nevertheless groundbreaking. It links two quantities that otherwise only describe waves or particles. The wavelength λ describes the distance between two crests of a wave. The momentum p, on the other hand, describes the product of the mass and velocity of a particle. h is a natural constant deeply rooted in quantum physics: Planck’s constant. This formula has far-reaching consequences: It says that every particle that weighs something and moves also has a wavelength. It predicted the existence of matter waves.
What does it mean? It means: the electron (for example) is not only a particle but also a wave. Just like light is not only a wave but also a particle. This principle is one of the cornerstones of quantum physics and it is commonly called wave-particle dualism because it works both ways.
Winners and losers
The wave property of the electron is not just a mathematical tomfoolery; it can be observed experimentally. And here we come back to the double-slit experiment – the experiment that only works with waves. And indeed: In 1927, the two physicists Clinton Davisson and Lester Germer of Bell Labs succeeded in observing the interference of electrons. They shot electrons at a grating (which in principle does exactly the same thing as a double slit) and they saw an interference pattern, just like for a wave!
Surprisingly, at almost the same time, someone else, namely the Brit George Thomson, did the same experiment. It is amazing that two independent groups are doing exactly the same research at the same time but it happens more often than you think. The outcome is often unpredictable. In this case, Thomson and Davisson shared the Nobel Prize for the discovery. And Germer? He is considered a tragic example of the arbitrariness of the Nobel Prize [1]. He was nominated for a Nobel Prize a total of 26 times together with his colleague Davisson, and still came away empty-handed in the end. That surprised pretty much everyone at the time. Was it because he was initially “only” Davisson’s assistant?
Perhaps it was also the well-known Thomson family name as George Thomson is the son of Joseph Thomson, Nobel Prize winner and discoverer of the electron. He was not thoroughly successful, however, as his plum pudding model lost against Rutherford’s atomic model. However, I would like to point out the irony that the father showed that the electron is a particle while his son proved that electrons behave like waves. Disagreements exist even in the best of families.
Interference with cats
So the photon is no longer alone in its identity crisis because the electron has also been shaken to the foundations of its beliefs. However, we have found out that the two – as different as they may seem – have a lot in common. And not only the two of them: de Broglie’s formula says that every particle is basically a wave.
Every particle? Let’s see about that! Since physicists have known that interference also works with particles, they have tried to take it to the extreme. Electrons, atoms, molecules – nothing is safe any more. The largest particle with which interference has been observed so far was a molecule with about 2000 atoms [2]. The only problem is that the wavelength becomes smaller and smaller the heavier the particle, and this makes the actual realisation of the experiment extremely difficult.
But even 2000 atoms are still pretty small from our perspective. What about something bigger, like… a cat? No problem: purely theoretically, a strolling cat has a wavelength of about 10-33 m (that’s 32 zeros after the decimal point and before the 1). That’s pretty damn small, to say the least. And I am saying that as a quantum physicist who studies light particles. But even if a cat’s wavelength doesn’t matter in everyday life, it can still serve as useless party knowledge. And if you accidentally try to impress a dog person with this cat knowledge, remember that opposites are not always mutually exclusive.
Do you like what you read? If you don’t want to miss any new posts, don’t forget to subscribe to my blog.
References
[1] How to almost win the physics Nobel
[2] Quantum superposition of molecules beyond 25 kDa